
Research
One of the most important challenges of our time is the resource-conserving use of substances and materials. In this context, the development of new industrial processes has a major responsibility. For preparative chemistry, it is necessary to realize previously unknown synthetic routes and to use materials that meet high ecological and economic requirements. The focus here is on environmental compatibility and its correlation to process and energy costs. The latter are essentially determined by the availability of a substance or an element.
Numerous catalytic reactions use platinum groups metals such as ruthenium, rhodium, palladium, osmium, iridium and/or platinum. Without these metals, modern organometallic chemistry, preparative organic chemistry, and a variety of industrial processes would not be feasible. They have had a lasting impact on the chemistry of the 20th century and the beginning of the 21st century. However, their unlimited use for future applications and generations proves to be problematic since the aforementioned late transition metals are only available in limited quantities in the earth’s crust.
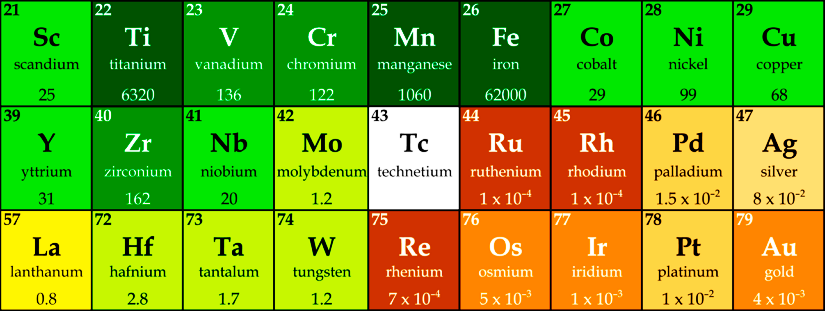
In contrast to the late transition metals mentioned above, the elements of the early transition metals (groups 3–5) are very abundant on Earth. Unfortunately, although these metals are already used in some very important chemical catalytic reactions, such as asymmetric epoxidation or olefin polymerization, they have so far shown a much lower reaction diversity compared to the platinum group metals.
Our research group is highly interested in expanding the reaction variety of the early transition metals. Here, the main focus is on the use of redox-active ligands which increase the redox capability of the early transition metal centers and at the same time stabilize the metal atom. In this way, we develop novel catalytic reactions with individual reaction steps, which would not be accessible without the use of this type of ligand. By “masking” low-valent metal centers, the jump between the individual oxidation steps is buffered to overcome the obstacles of the metal-centered redox reaction.
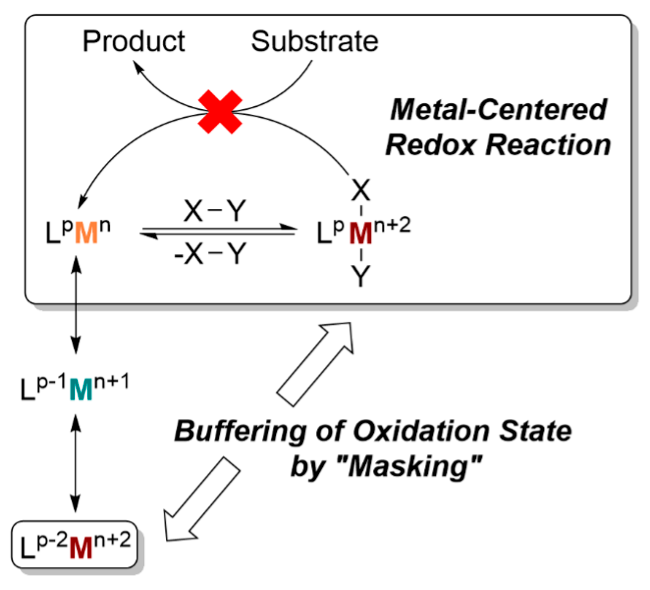
Here, the focus is not only on mononuclear complexes, but on bimetallic compounds of this type. Thus, in addition to questions of metal-ligand interaction, any metal–metal interactions and their consequences for subsequent chemistry can be studied in particular.
To find out more, check our publications subpage (soon 😉)
